Powered for Precision
Revitope is transforming the way immunotherapeutic drugs are designed. As experts in antibody engineering with a deep understanding of the cellular processes underlying immune-based drugs, we are leveraging biological insights and proven protein modules to design “gated” immunotherapies – medicines with sophisticated built-in control mechanisms for precision tumor targeting and controlled immune activation.
with Immunotherapy
of Possibilities
Technology Platform
Using our TwoGATETM Technology Platform, we are building a pipeline of novel cancer immunotherapies designed to elicit a focused and powerful immune response to eradicate tumor cells while leaving healthy tissue unharmed.
| Status | ||||||
|---|---|---|---|---|---|---|
| Programs | Indication | Discovery | Preclinical | IND | Phase 1 | Product Rights |
| TwoGATETM Targets undisclosed |
Solid Cancers | Junshi/Revitope | ||||
| REV-403 TwoGATETM EGFR x PDL1 |
Solid Cancers | Revitope | ||||
| TwoGATETM Targets undisclosed |
Solid Cancers | Junshi/Revitope | ||||
| TwoGATETM Targets undisclosed |
Solid Cancers | Genmab | ||||
| TwoGATETM Targets undisclosed |
Solid Cancers | Genmab | ||||
| TwoGATETM Targets undisclosed |
Solid Cancers | Genmab | ||||
| TwoGATETM Targets undisclosed |
|||
|---|---|---|---|
| Program | Indication | Product Rights | |
| TwoGATETM Targets undisclosed |
Solid Cancers | Junshi/Revitope | |
| Status | |||
|
Discovery
Preclinical
IND
Phase 1
|
|||
| REV-403 TwoGATETM Targets undisclosed |
|||
|---|---|---|---|
| Program | Indication | Product Rights | |
| REV-403 TwoGATETM EGFR x PDL1 |
Solid Cancers | Revitope | |
| Status | |||
|
Discovery
Preclinical
IND
Phase 1
|
|||
| TwoGATETM Targets undisclosed |
|||
|---|---|---|---|
| Program | Indication | Product Rights | |
| TwoGATETM Targets undisclosed |
Solid Cancers | Junshi/Revitope | |
| Status | |||
|
Discovery
Preclinical
IND
Phase 1
|
|||
| TwoGATETM Targets undisclosed |
|||
|---|---|---|---|
| Program | Indication | Product Rights | |
| TwoGATETM Targets undisclosed |
Solid Cancers | Genmab | |
| Status | |||
|
Discovery
Preclinical
IND
Phase 1
|
|||
| TwoGATETM Targets undisclosed |
|||
|---|---|---|---|
| Program | Indication | Product Rights | |
| TwoGATETM Targets undisclosed |
Solid Cancers | Genmab | |
| Status | |||
|
Discovery
Preclinical
IND
Phase 1
|
|||
| TwoGATETM Targets undisclosed |
|||
|---|---|---|---|
| Program | Indication | Product Rights | |
| TwoGATETM Targets undisclosed |
Solid Cancers | Genmab | |
| Status | |||
|
Discovery
Preclinical
IND
Phase 1
|
|||

- Genmab secures rights to Revitope’s proprietary, conditional T Cell Engagement (TCE) TwoGATE™ technology for conducting research against multiple drug target pairs with options for exclusive worldwide development and commercialization for up to three resulting products
- Revitope to receive an upfront payment of USD $9 million with milestone and fee payments up to USD $600 million plus tiered single-digit royalties on sales of successfully commercialized therapies
BOSTON, USA; 29 October, 2024 — Revitope Oncology Inc. (Revitope), a biotechnology company advancing a new class of precision cancer immunotherapies, announced today that it has signed a license agreement providing Genmab A/S (Genmab) access to Revitope’s conditional TCE technology, TwoGATE™. Genmab is granted exclusive rights to utilize TwoGate™ for multiple drug target pairs during a multi-year research period, including the option to take up to three exclusive licenses for worldwide development and commercialization of the resulting products.
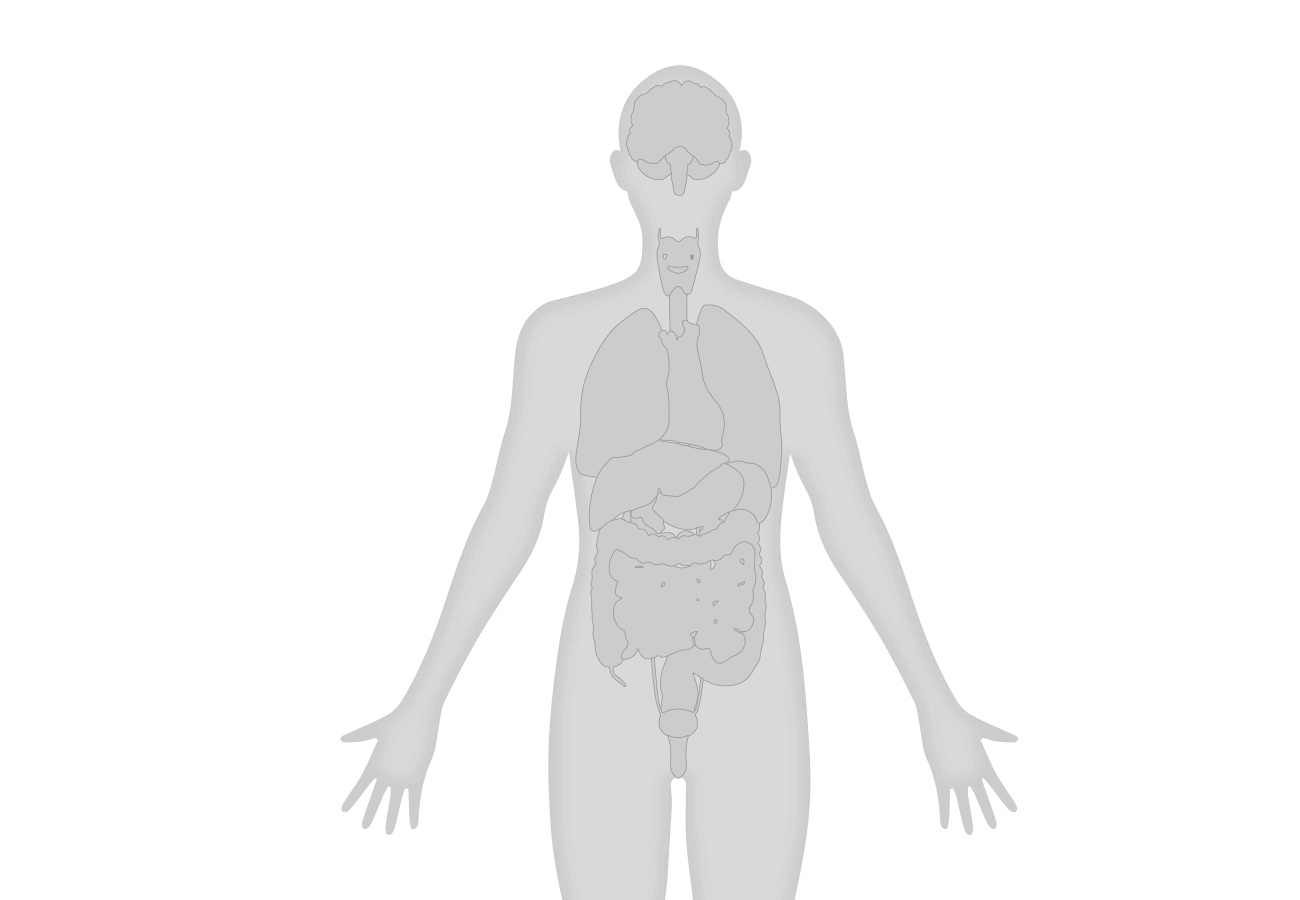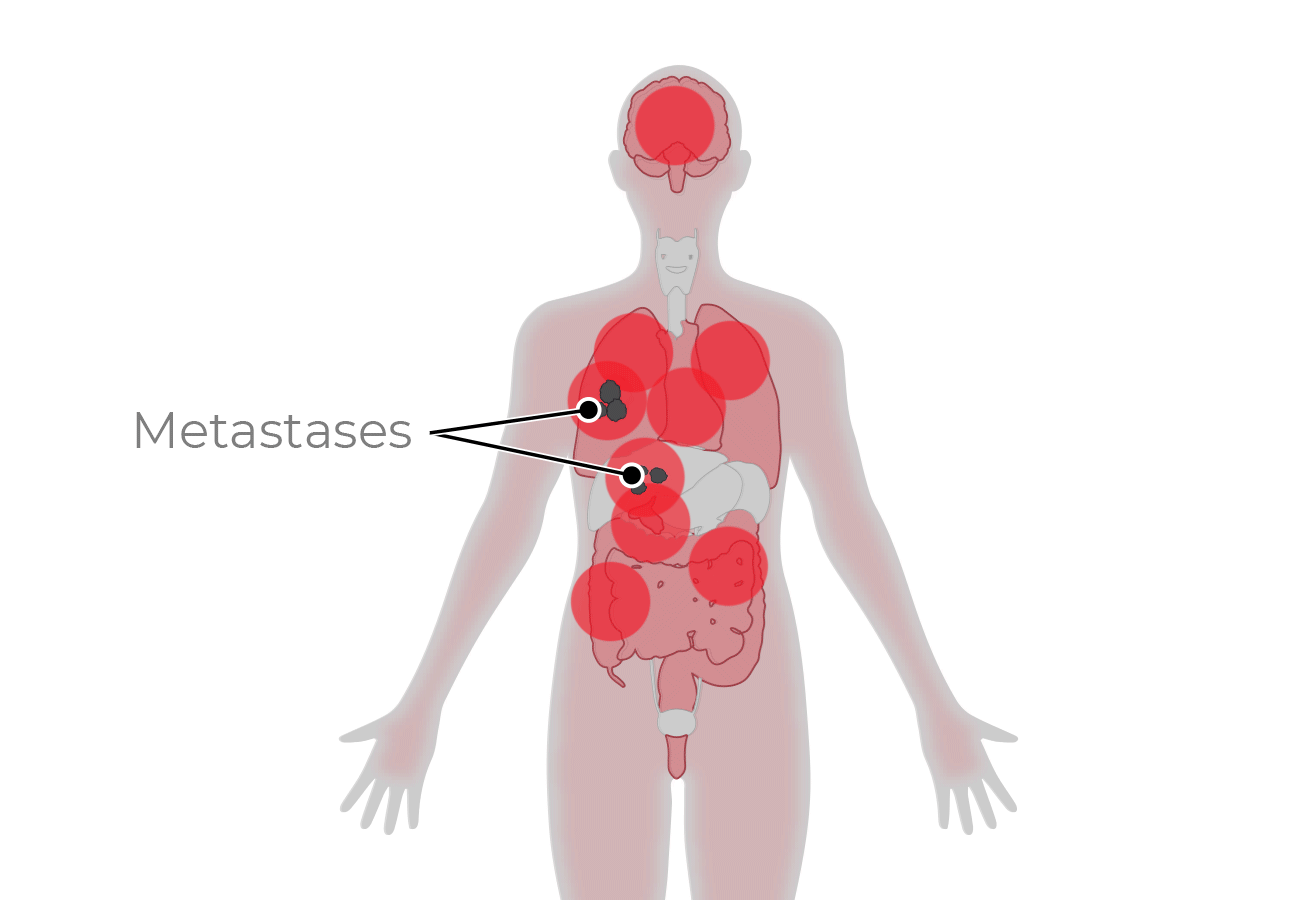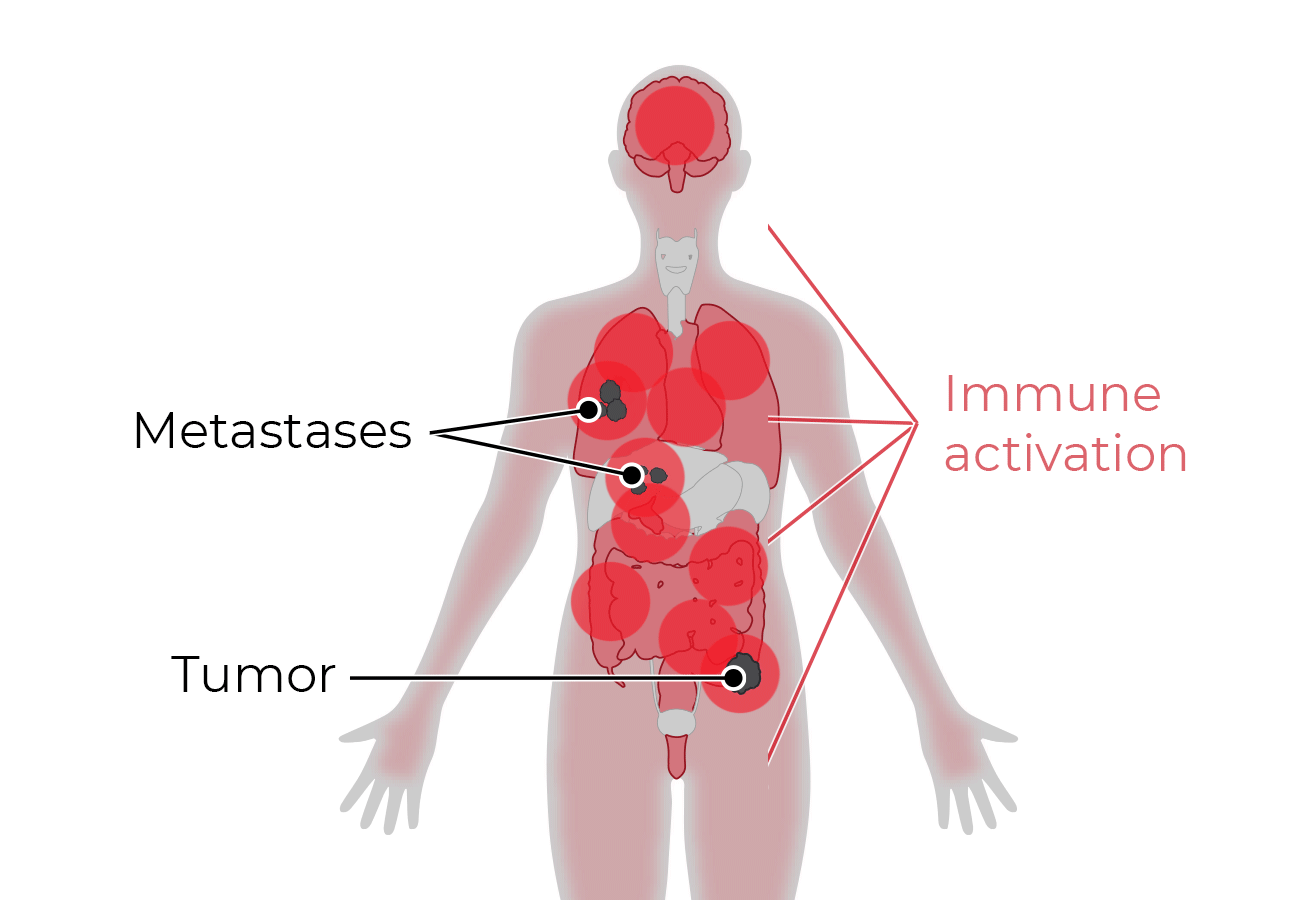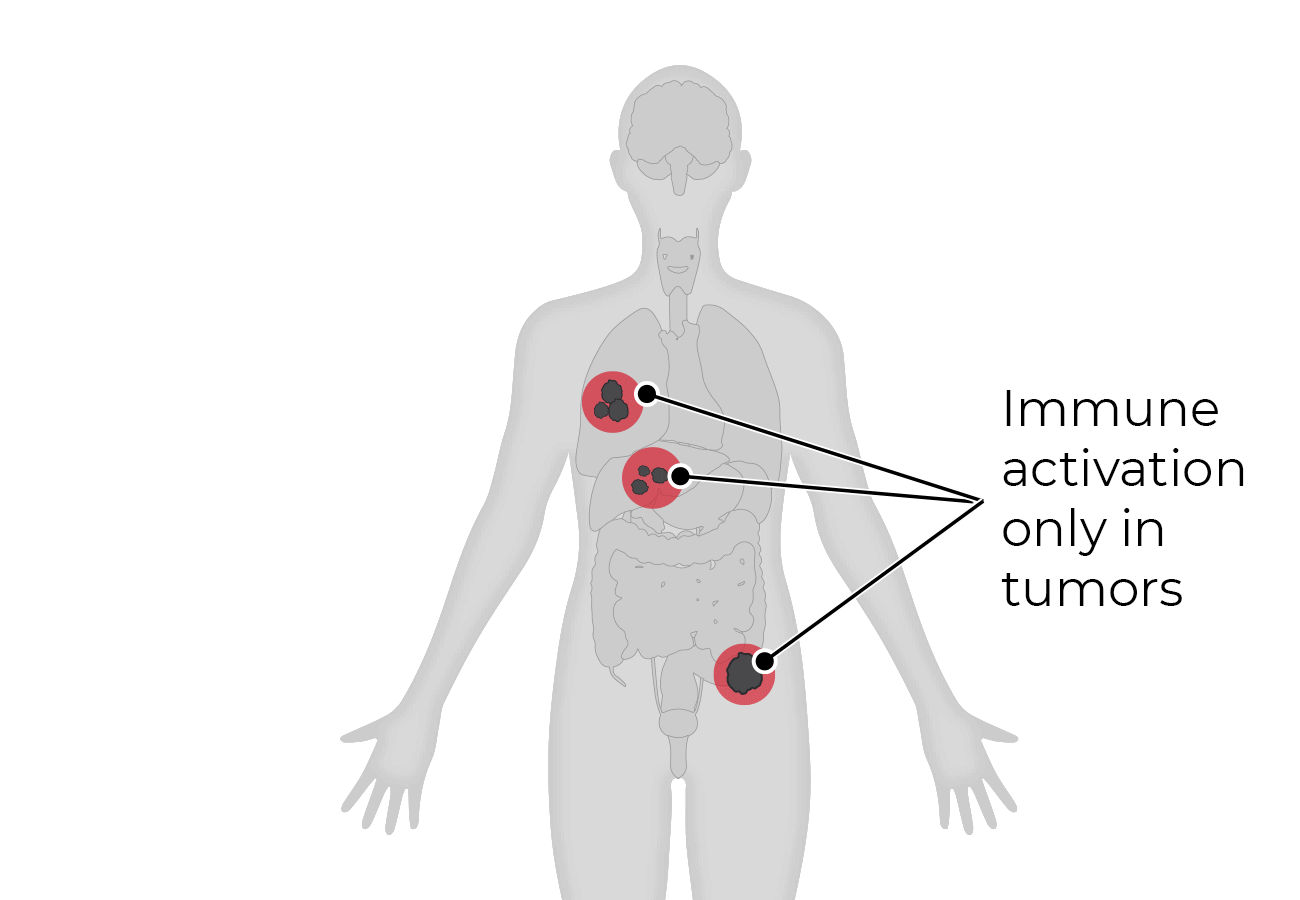
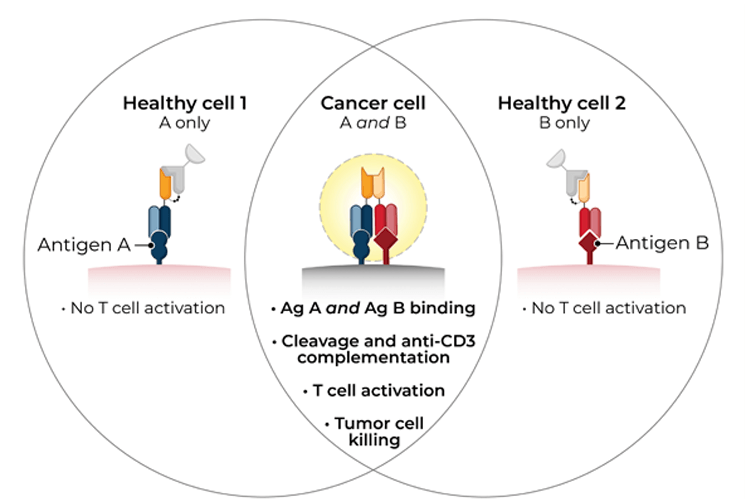
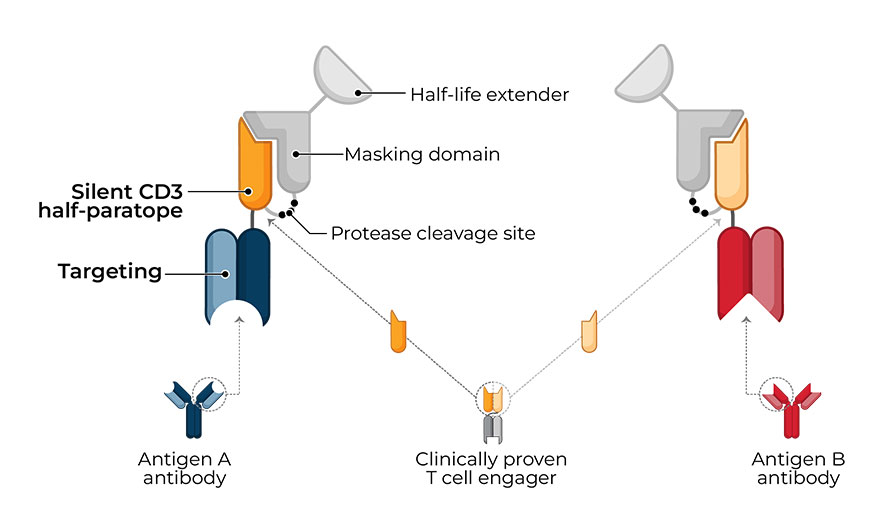
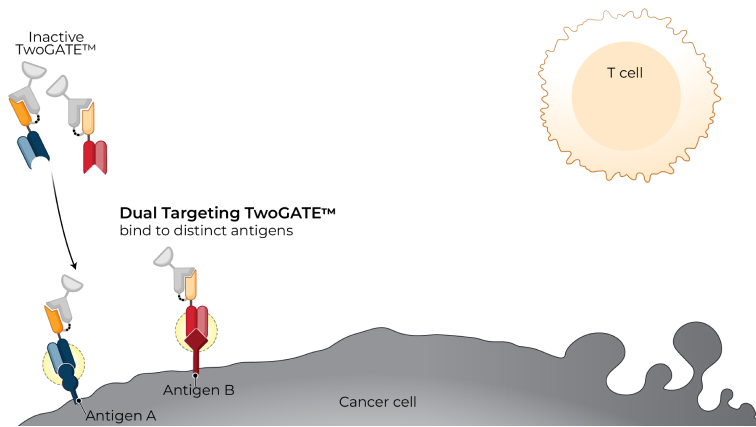
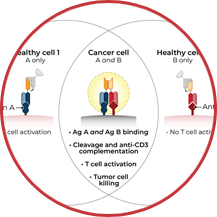
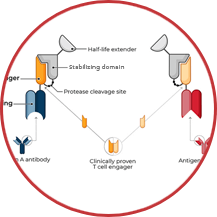
TwoGATE™ leverages the dual antigen binding requirement of a unique split paratope that assembles on the tumor cell surface to potently engage T cells with high precision, potentially addressing key areas of unmet need in the treatment of solid tumors.
“The unique blending of precision protein engineering in TwoGATE™ with target pairs that are exquisitely tailored to solid tumors with high unmet need, offers the potential to transform treatment of solid tumors by allowing TCEs to be dosed at levels that achieve significant efficacy without inducing systemic toxicities,” said Werner Meier, Chief Scientific Officer of Revitope. “Genmab is the ideal partner to bring this potential to patients.”
“We are thrilled to partner with Genmab, a leading biotechnology company,” said Mark Clement, Chief Operating Officer of Revitope. “In deploying our cutting-edge TwoGATE™ technology platform together with Genmab’s robust antibody engineering and development capabilities, Revitope aims to strengthen Genmab’s pipeline, thereby aiding the transformation of cancer treatment.”
Financial Terms
Revitope will receive an upfront payment of USD $9 million and, on a target pair-by-target pair basis, is eligible to receive option-exercise fees and development, regulatory and commercial milestone payments up to USD $600 million -if all three options are triggered- plus tiered, single-digit royalties on commercial sales.
About Revitope
Revitope is a privately funded biotechnology company with a focus on innovative tumor-specific biotherapeutics. Based in the Boston Massachusetts Biotechnology hub, the company has conceived, engineered, patented and pre-clinically tested novel classes of dual antigen targeting bispecific antibody therapeutics designed to enable tumor-specific immunotherapy with improved therapeutic efficacy and safety. For more information, please visit www.revitope.com or alternatively please contact us at contact.revitope@revitope.com
About Revitope’s TwoGATETM TCE Platform
Revitope’s proprietary TwoGATE™ TCE technology platform exploits unique combinations of antigens co-expressed on cancer cells to enable the development of highly specific cancer immunotherapies with improved safety and efficacy. The company’s unique approach combines a pair of tumor-targeted antibodies each carrying half of a silent T cell engaging domain that assemble and become active only when bound to cancer cells co-expressing both antigens. This allows for highly selective dual antigen targeting to elicit and focus a powerful gated immune response to tumor cells.

Revitope Oncology, Inc. is a privately owned company in Cambridge, MA, that focuses on the development of next-generation T cell engager immunotherapies for a variety of solid cancer indications. Revitope’s platform is a suite of proprietary and modular bispecific antibodies designed to deliver improved therapeutic efficacy and safety through built-in control mechanisms that enable exquisite tumor-specificity. The company has several cancer programs in preclinical development and expects to have its lead program in the clinic by early 2023.

Related Links

CONCORD, Mass., Feb. 9, 2021 /PRNewswire/ — Applied BioMath (www.appliedbiomath.com), the industry-leader in applying systems pharmacology and mechanistic modeling, simulation, and analysis to de-risk drug research and development, today announced that Revitope Oncology is extending its collaboration to develop a systems pharmacology model for Revitope’s dual antigen targeting TwoGATE™ platform for solid tumor indications. “Our previous collaborations with Applied BioMath proved extremely valuable as we leveraged their models to identify optimal therapeutic parameters in our development process,” said Werner Meier, CSO of Revitope Oncology. “We look forward to extending our prior collaboration with this project to help accelerate our lead pipeline candidate towards the clinic.” In this collaboration, Applied BioMath will develop a semi-mechanistic systems pharmacology model to aid in the prediction of a human efficacious dose.
Applied BioMath employs a rigorous fit-for-purpose model development process which quantitatively integrates knowledge about therapeutics with an understanding of its mechanism of action in the context of human disease mechanisms. Their approach employs proprietary algorithms and software that were designed specifically for systems pharmacology model development, simulation, and analysis. “We are very excited to assist Revitope as they advance their therapeutic,” said Dr. John Burke, PhD, Co-Founder, President, and CEO of Applied BioMath. “Systems pharmacology models are increasingly necessary given the complexity of modern therapeutics. We look forward to extending our collaboration with the Revitope Oncology team.”
About Applied BioMath
Founded in 2013, Applied BioMath’s mission is to revolutionize drug invention. Applied BioMath uses mathematical modeling and simulation to provide quantitative and predictive guidance to biotechnology and pharmaceutical companies to help accelerate and de-risk drug research and development. Their approach employs proprietary algorithms and software to support groups worldwide in decision-making from early research through clinical trials. The Applied BioMath team leverages their decades of expertise in biology, mathematical modeling and analysis, high-performance computing, and industry experience to help groups better understand their candidate, its best-in-class parameters, competitive advantages, patients, and the best path forward into and in the clinic. For more information about Applied BioMath and its services, visit www.appliedbiomath.com.
Applied BioMath and the Applied BioMath logo are registered trademarks of Applied BioMath, LLC.
About Revitope Oncology, Inc.
Revitope Oncology, Inc. is a privately funded cancer therapeutics company with a focus on innovative tumor-specific antibody-based T cell engager immunotherapies. Based in Cambridge, MA, the company has conceived, engineered, patented and pre-clinically tested novel classes of bispecific antibody therapeutics designed to enable tumor-specific immunotherapy with improved therapeutic efficacy and safety. For more information, please visit revitope.com or contact us at info@revitope.com.
Revitope, the Revitope logo and TwoGATE™ are registered trademarks of Revitope Oncology Inc.
Press contact:
Kristen Zannella
kristen.zannella@appliedbiomath.com
SOURCE Applied BioMath, LLC

Related Links

CAMBRIDGE, Mass., February 2, 2021 — Revitope Oncology, Inc., a biotechnology company advancing a new class of precision cancer immunotherapies, today announced the appointment of biotechnology pioneer Louis Lange, M.D., Ph.D., to its Board of Directors.
“We are pleased to welcome Lou to the Revitope Board of Directors during this unprecedented time at our company as we continue to build upon the tremendous momentum established with our programs, people and partnerships,” said Steve Arkinstall, Ph.D., Chief Executive Officer of Revitope Oncology. “Lou brings an enormous expertise that spans from disease mechanisms and drug discovery to extensive leadership experience in the biopharmaceutical space, having founded and led numerous biotech companies through critical clinical and regulatory milestones. We’re delighted to have him on board as we continue to advance our precision therapies and capitalize on our PrecisionGATE™ technology platform.”
“Lou is a respected scientific and entrepreneurial leader with a deep and impressive background in academia as well as broad experience in executive leadership roles at a number of biotechnology companies. He has a proven track record of leading companies through successful partnerships, mergers and commercialization agreements and is an exceptional addition to the Revitope Board,” said Andrew Allen, M.D., Ph.D., Chairman and Founder, Revitope Oncology.
Dr. Lange is a physician scientist with deep experience in academic medicine and the biotech industry. He currently serves as a General Partner at Asset Management Ventures with a focus on leading healthcare innovation in numerous biotech, genomics, bioinformatics and imaging companies. Dr Lange founded and was Chairman/CEO of CV Therapeutics, Inc. for 19 years, leading the company thorough the commercial launch of Lexiscan and Ranexa®, a billion dollar first-in-class late sodium channel blocker and the first anti-anginal drug class approved in 30 years. He led the sale of the company to Gilead Sciences for $1.5 billion in 2019 and remained a part-time senior advisor to the CEO of Gilead for a decade. Afterwards he founded several other biotech companies and led two acquisitions, one to GE Healthcare and one to Audentes, where he became Lead Director. Prior to joining the biotech sector, Dr. Lange spent 22 years practicing academic medicine at Harvard University and Washington University. Dr. Lange received his bachelor’s degree from the University of Rochester and holds an M.D. and a Ph.D. in biological chemistry, both obtained from Harvard University.
“Revitope has developed a highly innovative approach that applies protein engineering to produce specific and effective cancer immunotherapies,” said Dr. Lange. “The team’s deep understanding of underlying immune mechanisms and cancer targeting have resulted in a technology with enormous potential to overcome the limitations associated with traditional cancer immunotherapies and improve targeting outcomes in solid tumors, a continuing challenge in cancer therapeutics.”
About Revitope Oncology, Inc.
Revitope Oncology, Inc. is a privately funded cancer therapeutics company with a focus on innovative tumor-specific antibody based biotherapeutics. Based in Cambridge, MA, the company has conceived, engineered, patented and pre-clinically tested novel classes of bispecific antibody therapeutics designed to enable tumor-specific immunotherapy with improved therapeutic efficacy and safety. The company’s Precision Guided Antibody Tumor EngagerTM (PrecisionGATETM) Technology Platform incorporates three unique control mechanisms to specifically target cancer cells and elicit a powerful immune response focused entirely on the tumor – minimizing toxicity risk and widening the therapeutic window of treatment. For more information, please visit revitope.com or contact us at info@revitope.com.
Media Contact:
Marites Coulter
Verge Scientific Communications
mcoulter@vergescientific.com
+1 415.819.2214
# # #

Revitope’s PrecisionGATETM Technology Platform is designed to develop antibody-based bispecific immunotherapies that minimize toxicity to widen the therapeutic window and provide more efficacious cancer treatments
CAMBRIDGE, Mass., January 5, 2021 – Revitope Oncology Inc. (Revitope), a biotechnology company advancing a new class of precision cancer immunotherapies, today announced that the company has entered into a collaboration with Janssen Biotech, Inc. (“Janssen”), one of the Janssen Pharmaceutical Companies of Johnson & Johnson, evaluating Revitope’s proprietary T cell engager technology platform to develop next generation bi-specific antibody therapies. The agreement was facilitated by Johnson & Johnson Innovation.
“We are excited to enter into this collaboration with Janssen and employ Revitope’s PrecisionGATE technology platform to advance the development of T cell engager therapies with the aim of delivering safer, more efficacious therapies to patients,” said Steve Arkinstall, Ph.D., Chief Executive Officer, Revitope Oncology. “This marks our second collaboration in the last 6 months as we continue to accelerate the potential of our platform and generate more effective targeted cancer therapeutics.”
Revitope’s proprietary Precision Guided Antibody Tumor EngagerÔ (PrecisionGATE) technology platform exploits co-expressed tumor antigens to enable the development of highly specific cancer drugs with improved safety and efficacy over conventional immunotherapeutic approaches. The company’s unique approach combines a pair of tumor-targeted antibodies with a shared silent T cell engaging domain that become active only when they encounter cancer cells co-expressing both antigens. This allows for highly selective dual-antigen targeting to elicit and focus a powerful gated immune response to tumor cells.
Under the terms of the collaboration, Revitope will collaborate with Janssen to conduct a feasibility study in the evaluation of Revitope’s PrecisionGATE T cell engager platform.
About Revitope Oncology, Inc.
Revitope Oncology, Inc. is a privately funded cancer therapeutics company with a focus on innovative tumor-specific antibody-based T cell engager immunotherapies. Based in Cambridge, MA, the company has conceived, engineered, patented and pre-clinically tested novel classes of bispecific antibody therapeutics designed to enable tumor-specific immunotherapy with improved therapeutic efficacy and safety. For more information, please visit revitope.com or contact us at info@revitope.com.
Contact Information
Marites Coulter
Verge Scientific Communications
+1 415.819.2214

CAMBRIDGE, Mass., September 16, 2020 — Revitope Oncology, Inc., a biotechnology company advancing a new class of precision cancer immunotherapies today announced the appointment of life sciences industry veteran Carsten Reinhardt, M.D., Ph.D., to its Board of Directors. Dr. Reinhardt is Managing Director and Chief Development Officer of Immatics Biotechnologies and one of the early pioneers of bispecific T cell therapy.
“I am honored to welcome Dr. Reinhardt to the Revitope Board. Carsten is an accomplished and highly regarded leader in immunotherapy with deep knowledge in tumor target identification and extensive experience bringing biological drugs into the clinic,” said Steve Arkinstall, Ph.D., Chief Executive Officer, Revitope Oncology. “We are thrilled to have his insight and wisdom on the Board as we advance our first-in-class precision cancer immunotherapies to the clinic and explore partnership opportunities to expand the reach of our novel therapeutics.”
“Carsten has held impressive leadership roles at several globally respected biopharmaceutical companies that have each had a major impact on the world of bi-specifics and T cell therapies. He is ideally suited to help advance Revitope’s strategic plan and vision,” said Andrew Allen, M.D., Ph.D., Executive Chairman and Founder, Revitope Oncology.
Dr. Reinhardt is a physician scientist who since 2010 has served as Managing Director and Chief Medical Officer and more recently as Chief Development Officer of Immatics, a German/U.S. company that has pioneered the field of tumor antigen identification (HLA-peptide complexes) and is now primarily focused on T cell therapy against these targets. While at Immatics, he has been intimately involved in successful fundraising including becoming public at NASDAQ as well as forging biopharmaceutical partnerships with Bristol Myers Squibb, GSK, Amgen, Roche and others. Prior to joining Immatics, he was Senior Vice President and Chief Medical Officer of Micromet, another pioneering German/U.S. company that drove the field of T-cell engager immunotherapies leading to the first ever FDA regulatory approval of a bispecific antibody (Blincyto™) and its acquisition in 2012 by Amgen for $1.6B. Dr. Reinhardt medicine at Ludwig-Maximilians University in Munich earning a Ph.D. at the Institute of Immunology in Munich in 1994. He has consulted to a variety of biopharma companies and is a visiting professor at the University of Basel in Pharmaceutical Medicine.
“Revitope has developed an incredibly thoughtful protein engineering approach with a platform that overcomes the inherent limitations and challenges of conventional immunotherapies,” said Dr. Reinhardt. “Despite the early success with some hematological cancers, finding specific surface targets for the treatment of solid cancers has been difficult and has hampered the successful translation of bispecific antibodies to the clinic. I strongly believe that the groundbreaking technology Revitope is developing has the potential to finally unlock the benefit of this treatment modality for patients with solid cancers who still desperately need efficacious treatment options.”
About Revitope Oncology, Inc.
Revitope Oncology, Inc. is a privately funded cancer therapeutics company with a focus on innovative tumor-specific antibody based biotherapeutics. Based in Cambridge, MA, the company has conceived, engineered, patented and pre-clinically tested novel classes of bispecific antibody therapeutics designed to enable tumor-specific immunotherapy with improved therapeutic efficacy and safety. For more information, please visit revitope.com or contact us at info@revitope.com.
Media Contact:
Marites Coulter
Verge Scientific Communications
+1 415.819.2214

- Novel antibody-based cancer immunotherapies to be developed through combining Revitope’s dual-antigen precision T-cell engager platform (TEAC) and Junshi Biosciences’ antibodies
- Revitope to design up to 5 TEAC molecules against tumor targets selected by Junshi Biosciences
- For each TEAC molecule selected, Revitope to receive up to $160M in development and commercialization milestones, plus tiered royalties on net sales
- Junshi commits to making a conditional equity investment of $10M for 9.99% of Revitope Oncology shares on an as-converted basis
Cambridge, MA & Shanghai, China | July 13, 2020 | Revitope Oncology Inc (“Revitope Oncology”)., a biotechnology company advancing a new class of precision cancer immunotherapies, its wholly-owned subsidiary Revitope Limited (Revitope Limited, together with Revitope Oncology, “Revitope”) and Junshi Biosciences (1877.HK, 688180.SH), a leading innovation‐driven biopharmaceutical company dedicated to the discovery, development, and commercialization of novel therapies, today announced the companies have entered into a strategic research collaboration. Revitope will leverage its proprietary protein engineering platform together with Junshi’s novel antibody components to develop first-in-class dual-antigen targeting cancer therapies. Revitope is granting Junshi a world-wide exclusive license on products arising from the research collaboration and will receive up to $160 million in development and commercialization milestone payments for each T Cell Engaging Antibody Circuit (TEAC) molecule selected by Junshi, plus tired royalties. Junshi also commits to making a direct equity investment in Revitope Oncology in the amount of $10M for 9.99% of total Revitope Oncology shares on an as-converted basis with terms and conditions to be mutually agreed and subject to compliance with all applicable laws.
“By leveraging Revitope’s unique two component T-cell immunotherapy platform and our in-house antibody capabilities reaching from discovery to commercialization, dual targeting precision-based novel cancer immunotherapies can be brought into clinical trials in the near future,” commented Dr. Sheng YAO, Vice President of Junshi Biosciences. “As an innovation-driven company, we believe the collaboration with Revitope will empower us to generate a new generation of first-in-class immunotherapy compounds designed to improve both safety and clinical efficacy.”
Revitope’s proprietary T Cell Engaging Antibody Circuit (TEAC) technology platform exploits co-expressed tumor antigens to enable the development of highly specific cancer drugs with improved safety and efficacy over conventional immunotherapeutic approaches. Revitope’s unique approach is based on a pair of tumor-targeted antibodies with a shared T-cell engaging domain which act as inactive pro-drugs unless they encounter cancer cells co-expressing both antigens.
“We are excited to partner with Junshi, a company with state-of-the-art antibody discovery technologies and world-class development capabilities, to advance our unique two-component T-cell engager therapies that have the ability to target tumor cells and deliver more efficacious and safer drugs to patients,” said Steve Arkinstall, PhD, CEO, Revitope Oncology.
Under the terms of the Collaboration and License Agreement, Junshi and Revitope will identify development candidate TEAC pairs against agreed upon targets. Revitope will leverage its TEAC protein engineering platform to develop up to five novel TEAC pairs using proprietary sequences from Junshi’s antibodies with best-in-class pharmacological and therapeutic activity. Junshi will receive a world-wide license to the TEAC pairs and will have sole responsibility for IND enabling studies as well as clinical development, manufacturing and commercialization. Revitope will receive up to $160M in clinical development and commercialization milestone payments for each TEAC molecule selected, plus tiered royalties on net sales.
About Revitope’s T-Cell Engaging Antibody Circuit Technology (TEAC): Tumor-specific Immunotherapies
Because tumors typically do not express cell surface proteins unique to the tumor, conventional bispecific antibody therapeutics can generate unwanted and substantial “on-target, off-tumor” toxicity. Revitope’s two-component T-cell engaging antibody circuits (TEACs) are designed to permit specific recruitment and activation of T-cells exclusively by tumor cells. Though developed with traditional tumor targeting domains, TEAC therapies split the CD3 paratope (the T-cell recognition domain) into two halves, with one half on one molecule and the other half on the other molecule. This allows for true dual-antigen targeting to a unique tumor-specific address – two inputs coming together to enable one precision targeted output i.e. a true “and” gate safety feature. Only when the two molecules come together through binding to their different tumor targets on the same tumor cell can the two halves of the CD3 binding domain recombine and create a fully functional anti-CD3 domain (a TEAC). Normal cells expressing only one or neither of the targeted antigens will not elicit activation of a TEAC pair thereby avoiding unwanted toxicity in healthy tissues.

Cambridge, MA. | January 8, 2020 | PRNewswire | Revitope Oncology, Inc., a cancer therapeutics company advancing a novel class of tumor-specific bispecific antibody therapies, today announced the appointment of Steve Arkinstall, D.Phil., as Chief Executive Officer. Dr. Arkinstall brings more than 30 years of experience in biopharmaceutical research and development across multiple diseases areas, and is a respected industry leader.
Most recently, Dr. Arkinstall was CEO of Elstar Therapeutics, a venture-backed biotechnology company developing multi-functional antibody-based therapeutics to treat cancer. He previously was Chief Scientific Officer at Kymab, an antibody therapeutics company founded in Cambridge, UK, prior to which he spent 16 years in progressively senior research leadership roles at EMD Serono, and associated entities in Europe and the United States.
“We are thrilled that a leader of Steve’s caliber has chosen to join Revitope at this exciting time in the company’s evolution,” said Andrew Allen, M.D., Ph.D., Chair of the Revitope Oncology Board of Directors. “Under the skilled direction of CSO and acting-CEO Werner Meier, the company has advanced its core T-cell engaging antibody circuit (TEAC) technology to successful in vivo studies. We must now select our first development candidates and advance into IND enabling studies as a prelude to clinical trials. Steve is a superlative, and experienced executive ideally equipped to lead us through these critical phases.”
Revitope’s TEAC technology consists of tumor-specific antibody circuits designed to focus immune activation to the cancer cell surface while expanding the antigenic targeting space on solid tumors, avoiding healthy tissues and widening the therapeutic index in immune-oncology.
“A key challenge for immunotherapy for solid tumors in particular, has been the absence of antibody-accessible antigens on the surface of tumor cells which are suitably specific to the tumor,” said Dr. Arkinstall. “Revitope’s “AND” gate technology, applied to antibody therapeutics, is an elegant and unique approach to the problem that offers a powerful solution. I am excited to be joining the team and look forward to driving this therapeutic approach forward to help cancer patients in a compelling new way. There is an abundance of targets to choose from and tremendous opportunity to leverage our technology through both in-house and partnered programs across cancer types.”

Concord, MA | July 10, 2018 | PRNewswire—Applied BioMath (www.appliedbiomath.com), the industry-leader in applying mechanistic modeling, simulation, and analysis to de-risk and accelerate drug research and development, today announced a collaboration with Revitope for an in-vitro and human mechanistic PK/PD modeling of Revitope’s bispecific T Cell Engaging Antibody Circuits (TEAC) targeting solid tumors. “TEAC are designed to increase tumor specificity and launch an immune activation only when bound to the cancer cell surface. This innovative engineering approach has the potential to unleash potent immune responses that are focused entirely on the tumor,” said Werner Meier, CSO and acting CEO of Revitope Oncology. “Our goal in this collaboration is to leverage Applied BioMath’s modeling and analyses capabilities to identify TEAC drug properties that drive the potential for a better therapeutic index and ideally more efficacy in immuno-oncology.”
Applied BioMath employs a rigorous fit-for-purpose model development process, referred to as Model-Aided Drug Invention (MADI), which aims to quantitatively integrate knowledge about therapeutics with an understanding of its mechanism of action in the context of human disease mechanisms. Their MADI approach employs proprietary algorithms and software that were designed specifically for mechanistic PK/PD modeling. “Our mechanistic modeling approach allows our collaborators to assess the feasibility of their therapeutic much more quickly than if they were to rely on experiments alone,” said Dr. John Burke, PhD, Co-Founder, President, and CEO of Applied BioMath. “They’ll be able to quickly answer strategic questions about the ideal properties for their therapeutic concept and help accelerate development in Lead Generation and by prioritizing experiments, thus helping them get into the clinical faster and for less money with potentially a BIC therapeutic, giving themselves a much higher chance of clinical success and maximizing R&D ROI.”
Paris, France & Cambridge, US | April 26, 2018 | GLOBE NEWSWIRE—Independent international pharmaceutical company Servier and LabCentral, a well-known launchpad for innovative startups in the United States, have announced that Revitope, a startup specializing in biotechnology, has won the Golden Ticket awarded by Servier as part of its sponsorship of the prestigious American coworking facility. The prize is a year’s residency at LabCentral’s facilities for a Revitope scientist. Located in Cambridge, LabCentral is at the heart of scientific and technological innovation in the United States.
Servier has been a Gold sponsor of LabCentral for two years. Each year, it can award a Golden Ticket to a startup in the field of healthcare in order to provide support for a research project corresponding to the Group’s development strategy.
“Servier is happy to have given this award to Revitope whose innovative technology could enable the therapeutic antibodies developed by the Group to precisely target the cells to be treated in patients with cancer and inflammatory diseases”, said Olivier Nosjean, Scientific Director of the Center of Excellence Research and Biopharmacy. “In an open-innovation approach, Servier is committed to supporting promising startups in the field of healthcare, and particularly oncology, one of the company’s priority areas of research.”
The Golden Ticket award underwrites the cost of a lab bench for a Revitope scientist to reside in LabCentral’s open lab for one year. This includes access to all the latest research equipment, shared infrastructure, extensive programming and services offered by LabCentral. The start-up will also benefit from LabCentral’s corporate development support (co-working area, business coaching, investor relations).
“A core principle in every aspect of what we do at LabCentral — from the design of our facilities and speaker series to our sponsorship programs and networking events — is a deliberate attempt to encourage interaction and collaboration,” said LabCentral Cofounder and President Johannes Fruehauf, M.D., Ph.D. “The Golden Ticket program is a great example. It’s enormously gratifying to see our mission come to fruition, with Servier helping to support Revitope’s continued work at LabCentral.”
Revitope Oncology is developing prodrug platforms that have the potential to unleash potent immune responses that are focused entirely on the tumor. The company’s programmable antibody circuits are designed to focus immune activation to the cancer cell surface while expanding the antigenic targeting space on solid tumors to widen the therapeutic index in immune-oncology.
Revitope Chief Scientific Officer Werner Meier, commented, “We are very excited to have been selected by Servier as their Golden Ticket winner for 2017. It is particularly gratifying that an international pharma leader like Servier — laser-focused on bringing innovative therapies to patients across important therapeutic areas including cancer — is recognizing the potential of the Revitope prodrug platforms to widen the therapeutic index for T-cell redirection. The Servier Golden Ticket provides much-appreciated support that helps us to continue to be part of the LabCentral entrepreneur community — a terrific place for young, life sciences companies to grow.”
The cooperation agreement with LabCentral is part of Servier’s dynamic collaborative partnership strategy which aims to better respond to the challenges of therapeutic innovation by placing its research activities in the most dynamic research ecosystems where creativity, innovation and entrepreneurship are promoted and valued. This new agreement also confirms Servier’s commitment to developing its partnership strategy in the United States
For general inquires please e-mail info@revitope.com.